
Therefore, the optimal therapy for these patients should be further investigated. Conclusion: The pCR rate after NAC in ER+/HER2-subtypes of breast cancer is low. We identified 22.5% as the best cut-off value for ki-67 expression in predicting complete response to NAC.
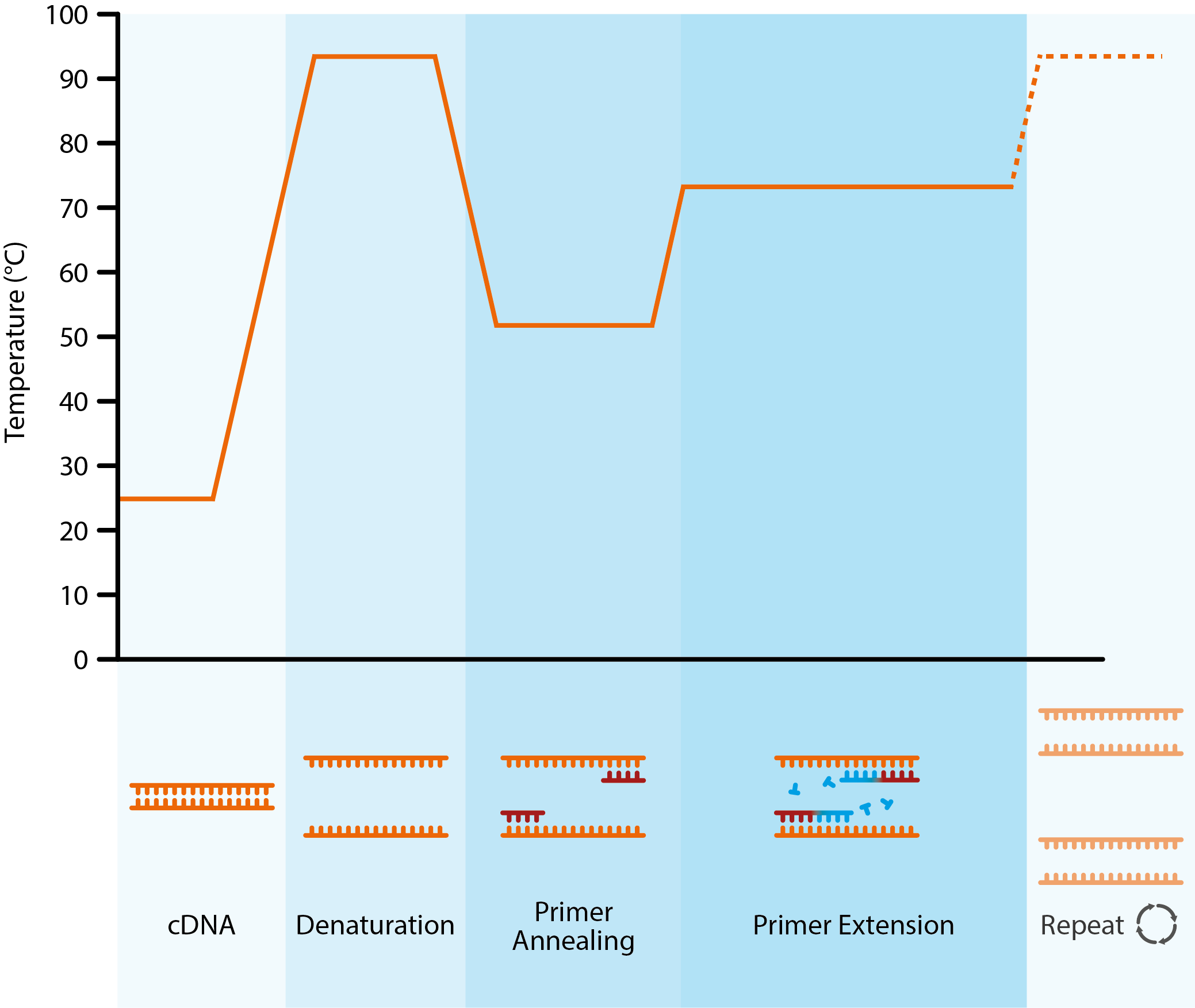
The overall pCR (both breast & node) was observed in 14.6% (n=46) of the 272 patients in which the data of breast and nodal were available. Nodal pCR was reported in 30.9% (n=97) of the 249 node-positive patients. Results: Breast pCR was seen in 25.2% (n=79) of the 314 cancer patients and partial response was seen in 47.8% (n=150), too. Meanwhile, receiver operating characteristic (ROC) curve analysis was performed to assess the predictive value for proliferative index (Ki-67%) expression. RAPIDS had favourable accuracy for the prediction of pathological complete response in the training cohort (AUC 0868 95 CI 08250912), and in validation. Breast and axillary lymph node pCR were assessed. Meanwhile, we sought to examine the impact of predicting factors on the rate of pCR Materials and Methods: In this multicenter retrospective study medical records data of 314 women with ER+/HER2- breast cancer subtype who received neoadjuvant chemotherapy was extracted from oncology centers' data between 20. Our objective was to evaluate the breast and nodal pCR in breast cancer patients with estrogen receptor-positive (ER) and HER2 negative subtypes. In fact, the events that pembrolizumab prevented most were distant metastatic recurrences.Abstract : Objective: The pathologic complete response (pCR) in the breast and axillary lymph node after neoadjuvant chemotherapy (NAC) would improve outcomes and it is used as a surrogate marker for survival. This means that we avoided in 20% of the patients, either local recurrence, distant recurrence, or progression during the neoadjuvant phase. If they were randomized to the pembrolizumab arm, the EFS at 3 years was 75% compared to only 56% in those who only received chemotherapy with the placebo. PCR (polymerase chain reaction) tests are a fast, highly accurate way to diagnose certain infectious diseases and genetic changes. The largest statistically clearly significant benefit was seen among those who are in the RCB2 category, which happens to be the largest subpopulation among those who do not achieve a complete response.Ġ:56 | That was approximately 20% of the entire study population on the pembrolizumab arm and the chemotherapy alone arm combined that had this RCB2 extent of residual disease. We see this by plotting their EFS by the various residual disease categories for those who had less than a complete response. Many patients who did not achieve pCR also benefited from pembrolizumab. According to Pusztai, the greatest difference in the arms was a reduced risk of distant metastatic recurrence with pembrolizumab.Ġ:08 | The most important finding from my presentation is that the benefit from pembrolizumab extended beyond just increasing the pCR rates.

Those with residual cancer burden scores of 2 (RCB2) which were the largest subpopulation that did not have a complete response, had a 3-year EFS rate of 75% with pembrolizumab versus 56% with chemotherapy alone, translating to an improvement in reduction of risk of local or distant recurrence, progression, or death. Pusztai says that the pembrolizumab benefited patients who had a pathologic complete response (pCR) but also appeared to benefit many who had a worse response to treatment based on their event-free survival (EFS).
Pcr complete response plus#
The phase 3 KEYNOTE-522 study randomly assigned patients with stage II or III TNBC 2:1 to receive neoadjuvant pembrolizumab or placebo plus chemotherapy, followed by definitive surgery, then adjuvant pembrolizumab or placebo.
Pcr complete response trial#
Lajos Pusztai, MD, DPhil, professor of medicine and coleader of Genetics, Genomics, and Epigenetics at Yale Cancer Center, discusses key takeaways from analysis of the KEYNOTE-522 (NCT03036488) clinical trial of pembrolizumab (Keytruda) plus chemotherapy in the neoadjuvant or adjuvant setting for patients with triple-negative breast cancer (TNBC).


 0 kommentar(er)
0 kommentar(er)
